Can Damaged Neurons Be Repaired
Neuroregeneration
-
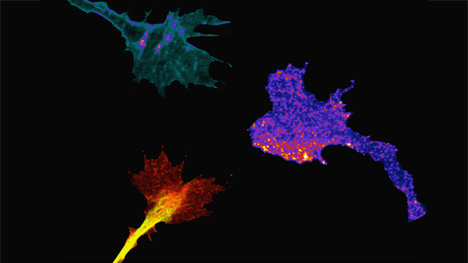
Spinal neuron growth cones
-
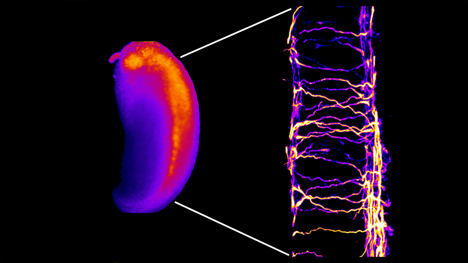
Fluorescent spinal neurons in the developing Xenopus embryo
-
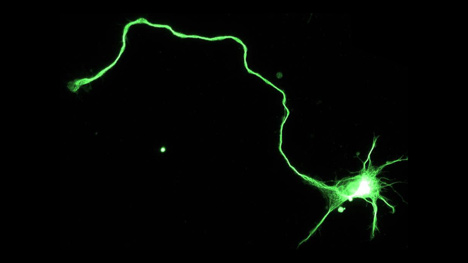
Hippocampal neuron immunostained to reveal dark-green microtubule cytoskeleton
-
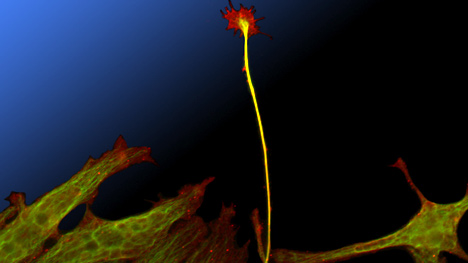
Nerve muscle co-culture
-
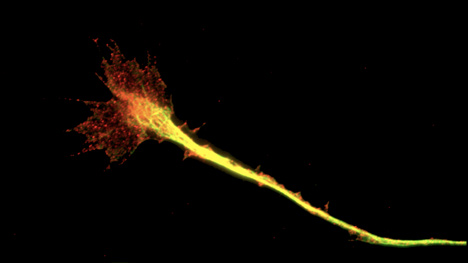
Contact adhesions in the nervus growth cone (paxillin in carmine, microtubules in greenish)
-
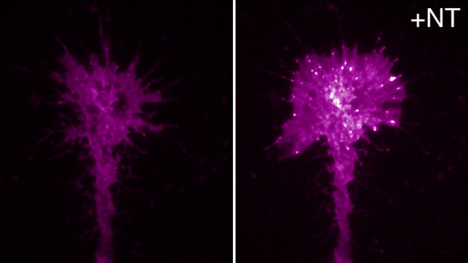
Substrate adhesions in the growth cone induced by brain derived neurotrophic factor
The circuitous, delicate structures that make up the nervous arrangement — the brain, spinal string and peripheral fretfulness — are susceptible to diverse types of injury ranging from trauma to neurodegenerative diseases that cause progressive deterioration: Alzheimer's illness, Parkinson's disease, amyotrophic lateral sclerosis (ALS, also known equally Lou Gehrig's disease), multiple sclerosis and multiple system atrophy.
Unfortunately, because of the complication of the encephalon and spinal cord, little spontaneous regeneration, repair or healing occurs. Therefore, brain damage, paralysis from spinal cord injury and peripheral nerve harm are often permanent and incapacitating.
Patients with serious nervous arrangement injuries or strokes oftentimes crave lifelong assistance, which puts a tremendous burden on patients, their families and club. Innovative, paradigm-shifting strategies are required to advance treatment of neurological injury. The neuroregeneration research at Mayo Clinic is at the forefront of healing the nervous system.
For an in-depth expect at neuroregeneration, see the neuroregenerative medicine at Mayo Dispensary booklet.
Focus areas
Mayo Clinic clinicians, scientists, engineers and other specialists in the Centre for Regenerative Medicine are taking a multidisciplinary integrative approach to neuroregeneration for a number of devastating neurological conditions. The inquiry is multifaceted, ranging from basic science discovery to clinical applications.
Affliction-specific research
-
Alzheimer's disease. Alzheimer'south disease is the major cause of dementia in older adults, with progressive loss of neurons in areas of the brain responsible for learning and retentiveness. Efforts in Alzheimer's disease inquiry focus on understanding why neurons degenerate in brains with Alzheimer's disease and how to either wearisome downwards the procedure or replace lost neurons.
Mayo researchers are investigating the effects of restoring cerebrovascular office, through transplantation of induced pluripotent stem (iPS) cell-derived vascular progenitor cells, on amyloid pathology and cognitive function in amyloid Alzheimer's disease model mice. The iPS cells converted from skin fibroblasts by transducing iv transcription factors (Oct3/iv, SOX2, Klf4, c-Myc) have the potential to generate all tissues in the trunk, including vascular cells.
This innovative approach will likely allow for rational designs of vascular regenerative therapy confronting Alzheimer'south disease.
- Amyotrophic lateral sclerosis. Mayo Dispensary researchers are testing a jail cell-based therapy for amyotrophic lateral sclerosis (ALS). All the same in its early stages, this enquiry uses adipose-derived mesenchymal stem cells from the patient's ain body. These cells are modified in the laboratory and delivered back into the patient's nervous system to promote neuron regeneration.
Anthony J. Windebank, 1000.D., and Nathan P. Staff, M.D., Ph.D., both neurologists and researchers at Mayo Dispensary, talk over the latest research into ALS treatments.
-
Multiple sclerosis. While scientists understand much about the damage that happens to fretfulness and their insulating sheath (myelin) during multiple sclerosis (MS) and how the immune system causes this damage, the exact reasons for the immune organisation attack are very poorly understood. The lack of understanding of the exact cause of MS is a claiming for the development of constructive therapies, and Mayo Clinic laboratories are working to improve understand this affliction.
Protecting nerves and myelin from damage, or repairing myelin after it's been damaged, as well holds potential for the treatment of MS. Injury to nerves and myelin tin be severe in MS and is the major cause of functional impairments. Notwithstanding, spontaneous repair of this impairment is sometimes observed in people with MS. Researchers in the Centre for Regenerative Medicine are actively engaged in developing therapies designed to stimulate this repair and thereby promote recovery of lost part.
Antibodies that demark to myelin and nerve cells and protect fretfulness from damage and stimulate myelin regeneration have been identified. A recent study also has found that regeneration of the myelin sheath can be stimulated by small, folded Dna molecules (aptamers).
- Parkinson'due south disease. Researchers are studying the genetic contribution to susceptibility to Parkinson'south affliction through the institution of a bank of peel and iPS cell lines from people with Parkinson's disease. Having a jail cell line like this provides the ability to generate the cells that die in neurodegenerative affliction, assuasive researchers to better empathise the genetic crusade of this condition and develop new treatments for these disorders in the future.
-
Multiple organisation atrophy. Multiple system cloudburst (MSA) is a progressive, fatal neurodegenerative disorder. The hallmark of the disease is glial cytoplasmic inclusions. The primary component of glial cytoplasmic inclusions is blastoff-synuclein. Aggregation of alpha-synuclein microfibrils leads to a concatenation of events, including microglial activation, inflammation, and glial and neuronal degeneration. The likely mechanisms involved include growth factor (BDNF, GDNF) deficiency, toxic cytokines and oxidative injury.
Research focuses on the prevention of blastoff-synuclein aggregation past drugs such as rifampicin or paroxetine; the use of mesenchymal stem cells to provide and evangelize growth factors; and attacking microglial activation and the inflammatory response by agents such equally intravenous immunoglobulin.
Clinical treatments
-
Immune response and neuroregeneration. Researchers in the Mayo Clinic Center for Regenerative Medicine are developing numerous approaches to attenuate specific allowed cell types in central nervous system (CNS) inflammation and applying strategies to a multifariousness of diseases, including inflammation developing in the class of stalk cell transplant, cistron therapy or factor-driven regeneration of CNS tissues.
Studies take demonstrated a therapeutic issue in reducing motor dysfunction and blood-encephalon barrier disruption in model systems of multiple sclerosis through the removal of antigen-specific CD8 T cell responses. By optimizing the imaging of neuroinflammation with high-resolution confocal microscopy, small animal MRI and the profiling of CNS-infiltrating immune cells using flow cytometry, it'due south possible to isolate and phenotype CNS-infiltrating immune cells in vivo and visualize in real time the events leading to inflammatory devastation of nervous tissue.
-
Spinal cord repair. Regrowth of nerve fibers (axons) is essential to repair and functional recovery of the spinal cord. Tissue destruction with cysts and gliosis at the site of injury forms a barrier to regeneration.
Ongoing research is using tissue engineering with biodegradable polymer scaffolds (PLGA, PCLF, OPF) loaded with different growth-promoting cells (Schwann cells, neural progenitor cells, mesenchymal stem cells) and different growth factors (GDNF, NT3, BDNF) to bridge the gap, and to promote axonal regeneration and functional restoration in the spinal cords of rats and mice, eventually for futurity employ in patients.
Farther, Mayo Clinic researchers are investigating the effects of practise training and local commitment of steroids on axon regeneration and functional recovery.
-
Peripheral nervus regeneration and repair. The Center for Regenerative Medicine is developing strategies to expand the time window of opportunity and ameliorate the functional recovery following peripheral nerve injury and repair.
One strategy is to apply polymer microspheres to evangelize vascular endothelial growth factor (VEGF) to the nervus repair site in a controlled sustainable release manner. VEGF promotes angiogenesis and neurogenesis, and thus leads to a better functional outcome and larger window of opportunity for the nerve to be permissive to prolonged regeneration.
The other strategy is to counteract the lack of healthy Schwann cells at the nervus repair site by supplementing functioning Schwann cells derived from nerves prepared in an in vitro organisation or Schwann cells induced from stalk cells of the adipose tissue.
Novel brute models are beingness adult to delineate the nature and time course of denervation muscle changes; identify the key indicators of muscle receptivity, including electromyographic changes, muscle cobweb type changes and changes of myogenic genes; and evaluate the affect of these changes on nerve regeneration and the potential success of a nerve repair.
- Nerve prison cell regrowth: Axogenesis. Mayo researchers are using zebrafish as an animate being model organization to investigate how special cues in the brain and spinal cord can entice or block nerve cell growth — experiments that help scientists understand why conditions at the site of nerve injury retard regeneration. This piece of work is providing new understanding into how nerve cells grow during development of the nervous system and how nerve regeneration might be improved after injury.
-
Stroke neuroregeneration. Subsequently stroke, neurons near the penumbra are vulnerable to delayed only progressive damage as a result of ischemia. There is no effective handling to rescue such dying neurons. Researchers in the Eye for Regenerative Medicine hypothesized that mesenchymal stalk cells (MSC) can rescue damaged neurons later on exposure to oxygen-glucose deprivation (OGD) stress.
Studies have demonstrated that the MSC can differentiate into bone, cartilage and fatty tissues. Experiments in animal models of hemorrhagic stroke showed MSC therapy improves limb function. Taken together, this data will form the basis for using MSC to treat patients with contempo hemorrhagic stroke.
-
Neuro-oncology and neuroregenerative inquiry. Research currently focuses on invasive brain tumors (gliomas) for which patients receive a very poor prognosis. All the same, at that place are other brain tumors — oligodendroglioma and astrocytoma — that take a much ameliorate prognosis. Mayo Clinic researchers are interested in the mutations that are involved in the evolution of each of these unlike tumor types and why the tumors deport differently.
A target locus in a factor-poor region initially discovered past genome scanning has been identified. Research efforts are focused on studying the office of this alteration. Using mouse models, murine and man neural stem cells, and human induced pluripotent stem cells, Mayo researchers are investigating how the alteration modifies glial cell evolution.
-
Neuroregeneration and inflammation. The express capacity for repair in the nervous organisation is a significant medical claiming. The Center for Regenerative Medicine is developing new tools to effectively control the procedure of neural injury and degeneration and to create a microenvironment that enhances the capacity for innate repair and the efficacy of other regeneration strategies, including neural cell replacement and neurorehabilitation.
Research efforts focus on how highly druggable proteases (kallikreins) can exist targeted to prevent the complex cascade of tissue injury and abnormal reorganization that is a well-recognized component of CNS trauma — and which is increasingly recognized as an integral factor underlying the progression of many neurological disorders, including those classified equally neurodegenerative or neuroinflammatory as well equally those having an oncogenic basis.
Efforts are directed at agreement the physiological and pathophysiological consequences of a family of One thousand protein-coupled receptors (protease-activated receptors, or PARs), and determining whether PARs or the proteases that actuate them tin be targeted therapeutically to forbid pathogenesis and to promote CNS plasticity and repair to improve patient functional outcomes.
Methodologies
-
Deep brain stimulation for Alzheimer's illness. Anecdotal and initial trial reports concerning deep brain stimulation (DBS) to the fornix and hypothalamus accept been associated with improvement in memory function and reductions in expected cognitive pass up in patients with early Alzheimer'south illness. The fornix constitutes the major inflow and output pathway from the hippocampus and medial temporal lobe.
Mayo researchers have started an innovative airplane pilot study of dual-hemispheric stimulation of the subthalamic nucleus and fornix and hypothalamus to decide if this arroyo may have positive effects in attenuating cognitive turn down. If this study provides positive data, then the potential of using DBS of the fornix as a treatment for Alzheimer's disease will be considered.
-
Pediatric anesthesia, apoptosis and rubber. Exposure to multiple anesthetics at a immature historic period may exist associated with later problems, such every bit learning disabilities and attention-deficit/hyperactivity disorder. Researchers in the Center for Regenerative Medicine are working on a big project involving the detailed testing of 1,000 children to try to better ascertain what injury (if whatever) may be associated with anesthetic exposure. This information will be important to run into if this is really a problem in clinical do, and if and then, to alter practice to minimize problems.
Researchers are performing detailed neurodevelopmental testing on a sample from a birth cohort of children, including a testing bombardment previously used in primates shown to exist affected by anesthesia exposure. The aim is to ostend (or refute) prior findings and provide for the outset time a detailed phenotype of anesthesia-associated injury (if nowadays).
-
Neurogenesis. Past increasing the understanding of the molecular targets involved in regulation of adult hippocampal neurogenesis (neuron generation) and related behavioral responses contradistinct in neuropathological conditions, scientists can study underlying cellular and molecular mechanisms that regulate the production, maturation and integration of new neurons in the circuitry, and how aberrant neurogenesis plays a function in illness pathogenesis. Researchers are employing behavioral neuroscience to quantify cognition such as learning, retentiveness and feet.
Recognizing the therapeutic potential of developed neurogenesis, Mayo Clinic researchers are characterizing treatment systems and clinically approved medication that can let dictation of neuronal evolution in the correct direction. The long-term goal is to harness the regenerative capacity of adult neurogenesis toward an optimal clinical outcome and improved treatment options for brain disorders.
-
Neurorehabilitation. This research focuses on improving participation and the quality of life in people whose brain functions have been contradistinct by injury or disease. The focus is regenerative in that improved behavioral performance is possible only when adaptive anatomic and physiological change occurs within and between brain systems in response to therapeutic intervention.
By developing handling approaches that pb to improved function and independence, researchers in the Heart for Regenerative Medicine promote the adaptive regenerative changes in brain function that brand this improved behavioral operation possible.
-
Transduction mechanisms mediating bidirectional nerve growth. Cues released from the breakdown of myelin afterwards injury in the brain and spinal string may deed as chemorepellents and inhibit axon extension, which limits functional recovery. In contrast, positive cues such as neurotrophins tin can promote axon extension and elicit chemoattraction.
This research aims to determine how chemotropic cues in the microenvironment guide nerve growth and how dysfunctional guidance mechanisms can crusade disease. Understanding these mechanisms and discovering methods to dispense them are important for developing new therapies to promote neural regeneration later on degenerative disease or injury.
Researchers are determining how chemotropic cues in the microenvironment guide nerve growth and how dysfunctional guidance mechanisms can cause disease. This will permit scientists to define the spatiotemporal point transduction mechanisms by which nerve growth cones observe extracellular guidance cues and dynamically regulate cellular effectors to control the direction of axon extension during normal embryonic development and neural regeneration later injury.
Longer term, the research goal is to define mechanisms for priming and guiding regenerating axons to advisable synaptic targets to complete functional circuits.
More information
Neuroregenerative Medicine at Mayo Dispensary (PDF)
.
Source: https://www.mayo.edu/research/centers-programs/center-regenerative-medicine/focus-areas/neuroregeneration
Posted by: mellorateres1976.blogspot.com


0 Response to "Can Damaged Neurons Be Repaired"
Post a Comment